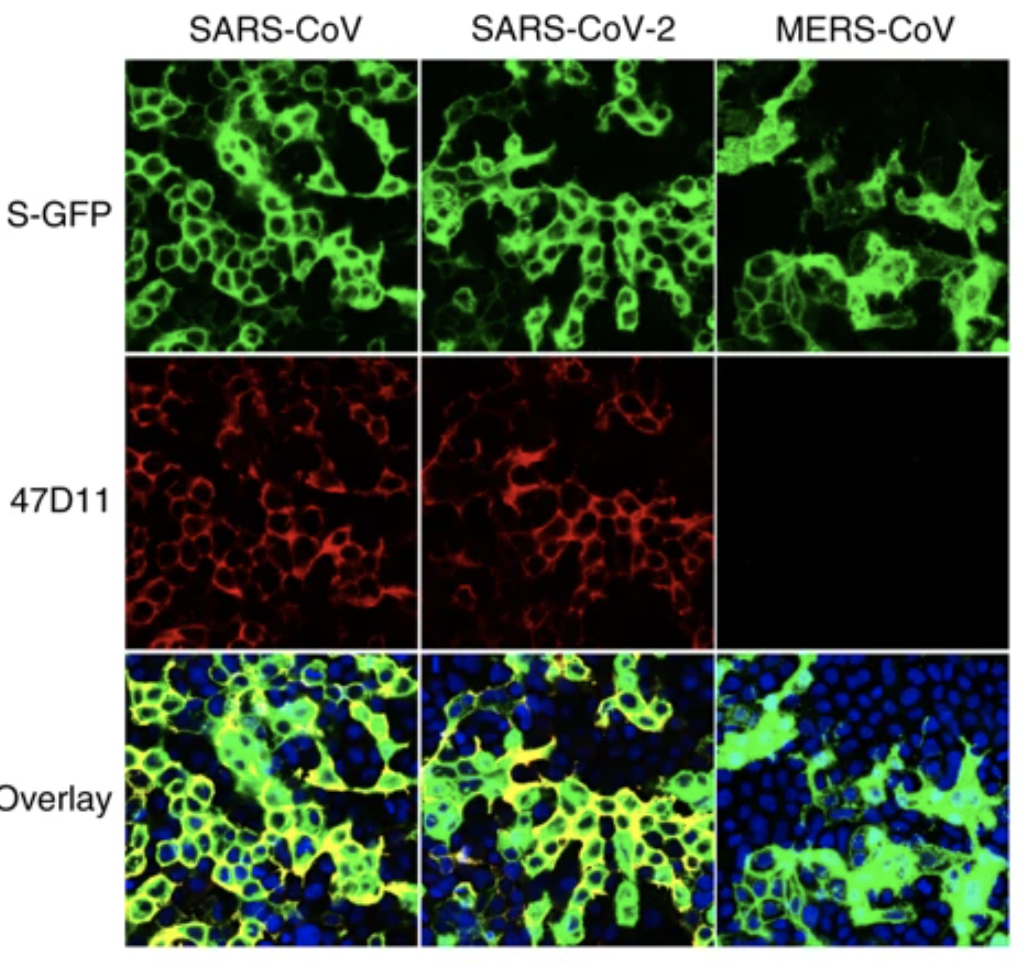
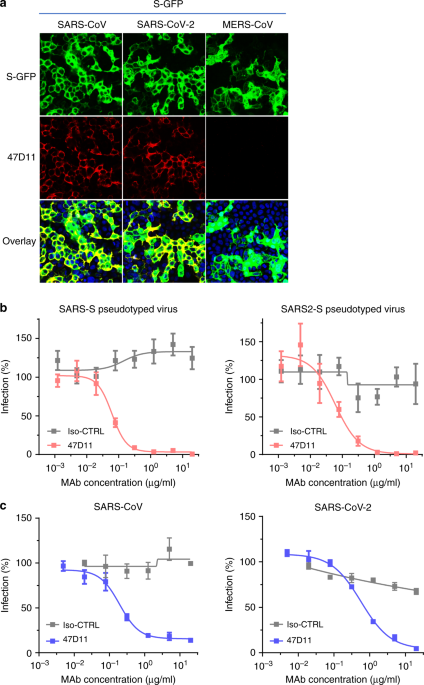
An article published in Nature Communications reports a recombinantly expressed human chimeric 47D11 H2L2 antibody, reformatted to a fully human immunoglobulin that binded to cells expressing the full-length spike proteins of SARS-CoV and SARS-CoV-2 and could potently inhibit infection of VeroE6 cells with SARS-S and SARS2-S pseudotyped VSV.
Abstract
The emergence of the novel human coronavirus SARS-CoV-2 in Wuhan, China has caused a worldwide epidemic of respiratory disease (COVID-19). Vaccines and targeted therapeutics for treatment of this disease are currently lacking. Here we report a human monoclonal antibody that neutralizes SARS-CoV-2 (and SARS-CoV) in cell culture. This cross-neutralizing antibody targets a communal epitope on these viruses and may offer potential for prevention and treatment of COVID-19.
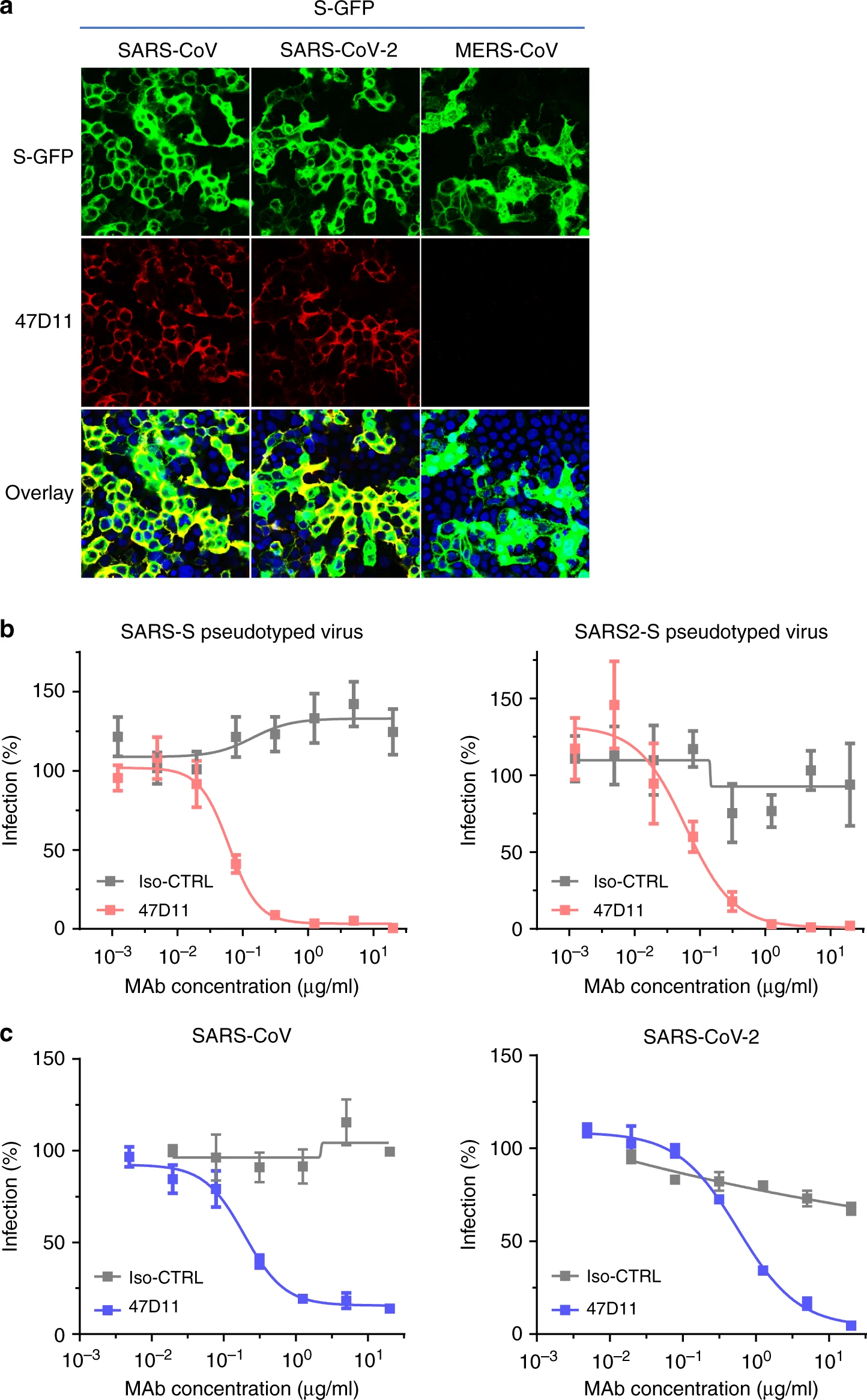
Wang, C., Li, W., Drabek, D. et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun11, 2251 (2020). https://doi.org/10.1038/s41467-020-16256-y
Further reading:
Potent human monoclonal antibodies against SARS CoV, Nipah and Hendra viruses
Abstract
Background: Recently, several potent human monoclonal antibodies (hmAbs) targeting the severe acute respiratory syndrome-associated coronavirus (SARS CoV) S glycoproteins, as well as the first fully human mAbs against two other paramyxoviruses, Hendra virus (HeV) and Nipah virus (NiV) have been discovered. Objective: To examine, compare and contrast the functional characteristics of hmAbs with potential for prophylaxis and treatment of diseases caused by SARS CoV, HeV and NiV. Methods: A review of relevant literature. Results/conclusions: Structural analyses have provided insights into the molecular mechanisms of receptor recognition and antibody neutralization, and suggested that these antibodies alone or in combination could fight the viruses' heterogeneity and mutability, which is a major problem in the development of effective therapeutic agents against viruses, including therapeutic antibodies.
Ponraj Prabakaran, Zhongyu Zhu, Xiaodong Xiao, Arya Biragyn, Antony S Dimitrov, Christopher C Broder & Dimiter S Dimitrov (2009) Potent human monoclonal antibodies against SARS CoV, Nipah and Hendra viruses, Expert Opinion on Biological Therapy, 9:3, 355-368, DOI: 10.1517/14712590902763755
https://www.tandfonline.com/doi/full/10.1517/14712590902763755